|

|
|
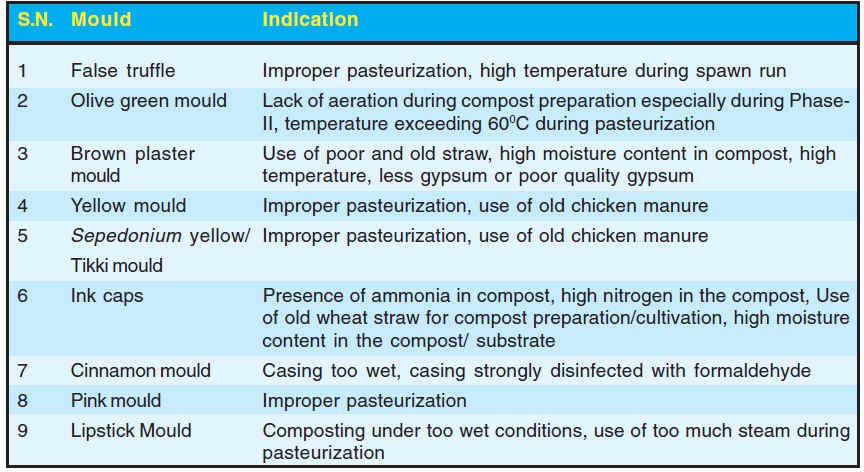
Fig. 1. Whitish, solid, wrinkled, rounded to irregular fungal masses of false truffle |
Fig. 2. Grey-green cockle-burns of olive green mould |
ii. Olive green mould (Chaetomium olivaceum, C. globosum)
The earliest sign of the fungus consists of an inconspicuous greyish-white fine mycelium in the compost or a fine aerial growth on the compost surface, 7-10 days after spawning. Frequently initial spawn growth is delayed and reduced. By late spawn run, fruiting structures that look like grey-green cockle-burns-1/16 inch in diameter (Fig. 2), develop on straw in isolated spots of the affected compost. The compost has a musty odour. Spawn usually grows into areas occupied by Chaetomium, although normal spawn growth is delayed. C globosum is also noticed in spawn bottles.
Epidemiology: The infection usually comes through air, compost and casing soil. It appears due to improper pasteurization accompanied by high temperature in the absence of adequate fresh air in phase-II. Loading to much compost in the tunnel having high moisture and bulk density not allowing proper penetration of air during Phase-II. This result in non-selective compost harbouring Chaetomium and other moulds. Spores are resistant to heat and are probably not killed easily during pasteurization. High moisture in compost results in the conversion of nitrogenous compounds into amines. Unfavourable conversions often result in renewed production of anhydrous ammonia, which prompts the growth of ammonia. Sometimes, the temperature is too high in certain spots of the tunnel, or may be less of oxygen, which often results in olive-green mould appearance. Ascospores are spread by air flows, clothes and other materials used in mushroom farm.
Control
● The fermentation period of the compost should not be too short. It is essential to achieve active compost that is not too wet and has a good structure.
● Do not add nitrogen sources like, ammonium sulphate, urea, chicken manure or similar materials just before filling.
● Compost should be properly pasteurized and conditioned with ample supply of fresh air. Higher temperatures (above 60°C) for longer time should be avoided.
● Sprays of Dithane Z-78 (0.2%) is recommended for the control of olive green mould.

|

|
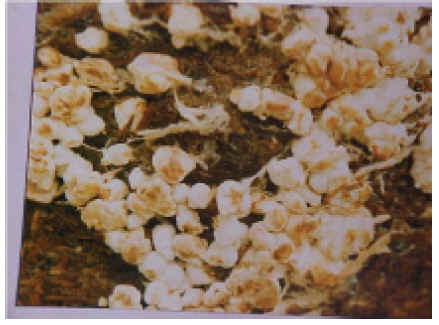
| Fig. 3. Brown plaster mould | Fig. 4. Yellow mould |
iii. Brown plaster mould (Papulaspora byssina)
It is first noticed as whitish mycelial growth on the exposed surface of compost and casing soil in trays as well as on sides in bags due to moisture condensation. This develops further into large dense patches gradually changing colour through shades of tan, light brown to cinnamon brown ultimately becoming rust coloured (Fig. 3). No mushroom mycelium grows on places where brown plaster mould occurs.
Epidemiology: Primary infection comes through air-borne bulbils or containers, compost and casing soil and workers. Its development is favoured by wet, soggy and wrongly prepared compost. Higher temperature during spawn run and cropping favours the disease development.
Control
● Composting should be carried out carefully using sufficient gypsum and not too much water.
● Peak heating / pasteurization should be for sufficient duration and at proper temperature. The compost should not be too wet before or after peak heating/ pasteurization.
● Localized treatment of infected patches with 2% formalin.
iv. Yellow mould (Myceliophthora lutea, Chrysosporium luteum, C. sulphureum)
Yellow moulds may develop in a layer below the casing (Mat disease), form circular colonies (Fig.4) in the compost (confetti) or they may be distributed throughout the compost (Vert-de-girs). In India, M. lutea is reported to induce mat disease. This fungus forms a yellow brown corky mycelial layer at the interphase of compost and casing, which is difficult to detect during the impregnation of casing layer by the spawn and even during the first break. It becomes apparent when it develops its stroma like morphology and mushroom production is severely inhibited.
Epidemiology: The major source of primary inoculum is air, chicken manure and spent compost. The secondary spread is mainly through mites, flies, water splashes, picking and tools. The fungus survives easily in the form of thick walled chlamydospores. Disease severity is generally more at high moisture content and 19-20°C temperature.
Control
● Properly pasteurized short method compost should be used.
● Proper pasteurization of the casing mixture is very essential.
● Bavistin (0.05%), blitox (0.04%) and calcium hypochlorite solution (15%) is effective for the control of this disease.
v. Sepdonium yellow/ Tikki mould: (Sepedonium spp.)
This mould is mainly observed in the compost and is initially white in colour turning to yellow or tan at maturity (Fig. 5). It is generally present in the lower layers of the compost or at bottom of the cropping bags. Various types of distortions in fruit bodies are commonly observed, probably due to the production of volatile substances or toxins. These toxins inhibit the spawn and ultimately mushroom mycelium disappears from the compost.
Epidemology: Primary sources of inoculum are soil, unpasteurized compost, spent compost, air or improperly sterilized wooden bamboos. The chlamydospore are thickwalled and resistant to heat. The fungus may also survive pasteurization temperature. Spores can spread to the compost by air currents prior to or during filling operation, during spawning operation or with unpasteurized or spent compost left in cropping room / sticking to bamboos under seasonal cultivation. Conditions favourable for button mushroom cultivation also favour the Sepedonium mould. Higher N content, especially in the form of chicken manure, has been reported to favour the mould development. Its appearance in the lower layers of the compsot has been linked with high moisture. Chicken manure harbours very high population of Sepedonium spp., which serves as the primary source of inoculum in long method compost.
Fig. 5 Tikki mould
C
ontrol● Strict temperature monitoring and control during compost pasteurization and an adequate post-crop cooking out are essential to eliminate the threat of infection.
● Preventing the entry of spores during spawning and spawn-running by installing high-efficiency air filters is essential.
● Incorporation of 0.5% carbendazim in compost and sterilizing chicken manure (for long method of composting) with 2% formalin or 0.5% carbendazim has shown good results.
● Treating finally prepared long method compost with 1.5 litre of formalin and 50 g of bavisitin per ton of ready compost and covering it with polythene sheets for 2 days prior to spawning almost eliminates the disease.
vi. Ink caps (Coprinus spp.)
Ink caps appear in the compost during spawn run or newly cased beds and outside the compost piles during fermentation. They are slender, bell-shaped mushrooms (Fig.6). Cream coloured at first, bluish-black later and are usually covered with scales. This fungus sometimes grows in clusters in beds and has a long sturdy stem, which often reaches deep into the compost layer. Several days after their appearance ink caps decay and form a blackish slimy mass due to autodigestion.

Fig. 6. Ink caps
Epidemiology: The infection generally comes through unpasteurized or improperly pasteurized compost or casing soil or air. Ink caps appear if the compost contains too much N, (too much chicken manure is used) or if the pasteurization period is too short. These are, therefore, genuine indicator moulds which indicate presence of NH3. Ink caps can also develop if insufficient gypsum is added to the compost or if peak heating has taken place at too low temperature or if the compost is too wet and poor in texture. Their incidence is also reported when old straw is used for composting. Ink caps can directly use free NH4 + and can also decompose cellulose very well, in addition to lipids and lignin. They are genuine coprophillic fungi, which have an optimum pH of around 8. The large masses of spores released through inking of the caps can very easily infect freshly prepared compost.
Control
● Use properly pasteurized compost and casing soil. Avoid excessive watering. Rogue out young fruit bodies of the weed fungus to avoid its further spread.
● Prepare compost using fresh straw.
● Ammonia in the compost at spawning should be less than 10 ppm i.e. no smell of ammonia.
vii. Cinnamon mould (Chromelosporium fulva, Perfect status Peziza ostrachoderma)
Its colour ranges from yellow gold to golden brown to cinnamon brown. The mould first appears as large circular patches of white aerial mycelium on the compost or casing (Fig. .7). Within few days the spores are formed and the colour changes from white to light yellow or light golden brown. As the spores mature, the colour changes to golden brown or cinnamon and the colony develops a granular appearance. Later the fungus produces numerous cup-like fleshy fruit bodies on beds (Fig. .8).
Epidemiology: Casing soil mixture and damp wood are the source of primary inoculum. Inoculum can blow through open doors or splash from floor during cleaning. The spores of the fungus are air-borne. Over pasteurized compost, over-heated patches during spawn run, high moisture content of the compost and excess of ammonia present in the compost favour the disease development.

Fig.7. White aerial mycelium of cinnamon brown mould Fig. 8. Cup-like fleshy fruit bodies of cinnamon brown mould
Control
● Casing soil should be properly sterilized by steam or formaldehyde. Newly cased beds should be sprayed with dithane Z-78 and maintain proper moisture content in casing layer.
viii. Lipstick Mould (Sporendonema purpurescens)
The disease first appears in spawned compost as a white crystalline mould, rather nondiscernable from spawn. As the spore of the mould mature, the colour changes from white to pink, to cherry red and then to dull orange or buff. White mycelial growth is more in loose areas of casing and can colonize well conditioned compost. In crops where there is a serious virus disease, lipstick mould usually occurs as a secondary disease.
Epidemiology: Soil, casing mixture and spent compost are the sources of primary inoculum. Water splashes or pickers further disseminate it. The mould is reported to be associated with the use of chicken manure in the compost.
Control
● Good hygiene is essential. Good pasteurization and conditioning of the compost will eliminate the pathogen.
ix. Pink mould (Cephalothecium roseum)
This mould appears as a white growth on the casing soil, which turns pink in due course. Infection generally comes through air. Mould can be checked by spraying thiram or captan (0.04%) twice on casing soil at 10 days interval.
x. Oedocephalum mould (Oedocephalum fimetarium)
This is a common mould observed on mushroom beds in Himachal Pradesh and incidence upto 60% has been observed in a farm at Solan during 1991. Artificial inoculation 162 Mushrooms : Cultivation, Marketing and Consumption of casing layer with O. fimetarium @ 5 g inoculum per 10 kg compost bag reduced the number and weight of fruiting bodies by 19.9% and 11.63%, respectively. The mould forms irregular, light silver gray patches on the compost surface during cooling before spawning. After spawning, the mould is light gray but changes to dark tan or light brown as the spores mature. Similar growth is also recorded on casing layer. Oedocephalum sp. in compost indicates that ammonia and amines were not completely eliminated during pasteurization and conditioning. Spraying or swabbing locally with 2% formalin controls the mould.
xi. White plaster mould (Scopulariopsis fimicola)
The mould appears as white patches on the compost or casing soil. These patches or mycelial mats may be more than 50 cm under favourable conditions. The white growth changes to light pink after a week of the formation of the spot. Spawn run is reduced significantly and under severe conditions complete crop failure is also recorded. The pathogen is favoured by under or overcomposting, which still retains the smell of ammonia and has high pH (>8.0). Proper composting and addition of optimum quantities of water and gypsum are recommended. Sprays of bavistin (0.1%) and local application of formalin (2%) after the removal of the mat are helpful in controlling the mould.
b. Fungal diseases
i. Wet bubble (Mycogone perniciosa)
Wet bubble produces two main symptoms, infected sporophores and sclerodermoid masses, which are the result of infection by M. perniciosa at different stages in the development of the sporophores. When infection takes place before the differentiation of stipe and pileus, the sclerodermoid (Fig. 18.9) is formed, whereas, infection after differetration results in the production of thickened stipe (Fig. 10) with deformation of the gills. The disease also results into white mouldy growth on the mushrooms, leading to their putrifaction (giving foul odour) with a golden brown liquid exudates. Cross section of deformed sporophores without cottony growth showed black circular area just beneath the upper layer.
Epidemiology : M. perniciosa spreads primarily through casing soil but the introduction of pathogen through other agencies, like spent compost and infected trash, can not be ruled out. The infection can be air-borne, water borne or may be mechanically carried by mites and flies. Water splash is an important factor for wet bubble spread on the beds. Spread through contact also occurres readily during watering and especially during harvesting. Chlamydospores have been reported to survive for a long time (upto 3 years) in casing soil and may serve as the primary source of inoculum. The aleurospores produced on the surface of monestrous structures are probably responsible for secondary infection.
Control
● Benomyl spray @ 0.1% immediately after casing has been reported to be very effective for protecting the crop. Application of carbendazim, chlorothalonil, prochloraz manganese complex (Sportak 50 WP) @ 0.1% into casing mixture have also been recommended for the management of wet bubble. A spray of 0.8 per cent formalin on to casing surface, immediately after its application on the beds is also effective.

Fig.9. Selerodermoid (Wet bubble infection)

|

|
| Fig. 10. Thickened stipe: wet bubble infection | Fig. 11. Typical onion shaped mushroom symptoms of dry bubble |
Use plastic pots/ cups to cover mushroom showing wet bubble symptoms during the cropping season to prevent spread of disease.
ii. Dry bubble (Verticillium fungicola)
This is the most common and serious fungal disease of mushroom crop. If it is left uncontrolled, disease can totally destroy a crop in 2-3 weeks. Whitish mycelial growth is initially noticed on the casing soil, which has a tendency to turn greyish yellow.
If infection takes place in an early stage, typical onion shaped mushrooms are produced (Fig. 11). Sometimes they appear as small undifferentiated masses of tissue upto 2cm in diameter. When affected at a later stage, crooked and deformed mushrooms with distorted stipes and tilted cap can be seen. When a part of the cap is affected hare-lip symptom is noticed. On fully developed fruit bodies, it produces localized light brown depressed spots. Adjacent spots coalesce and form irregular brown blotches. Diseased caps shrink in blotched area, turn leathery, dry and show cracks. Infected fruit bodies are malformed, onion shaped and become irregular and swollen mass of dry leathery tissue.
Epidemiology: The disease is introduced (primary infection) on to the farm by infected casing soil. Spread occurs by infected equipments, hands and clothings. The fungus is soil borne and spores can survive in the moist soil for one year. It also perpetuates through resting mycelium in spent compost. The optimum temperature for disease development is 20°C. The period from infection to symptom expression is 10 days for the distortion symptoms and 3-4 days for cap spotting at 20°C. The pathogen grows best at 24°C. High humidity, lack of proper air circulation, delayed picking and temperature above 16°C favours its development and spread. It becomes more common when cropping is extended beyond two months.
Control
Use of sterilized casing soil, proper disposal of spent compost and proper hygiene and sanitation are essential to avoid primary infection Two to three sprays of zineb (Dithane Z-78) @ 0.15% give good control of the disease. Carbendazim (bavistin) or benomyl (benlate) or thiophenate methyl @ 0.1% spray immediately after casing also control the disease. Application of prochloraz manganese complex (Sportak 50WP) @ 0.05%, 9 days after casing is also recommended for the management of the disease. Use of formaldehyde (2%) immediately after casing is also advocated for the effective management of dry bubble.
iii. Cobweb (Cladobotryum dendroides)
Cobweb appears first as small white patches on the casing soil, which then spreads, to the nearest mushroom by a fine grey white mycelium. A floccose white mycelium covers the stipe (Fig. 12), pileus and gills, eventually resulting in decomposition of entire fruit body. As the infection develops, mycelium becomes pigmented turning a delicate pink cover. In severe attacks, a dense white mould develops over casing and mushrooms change from a fluffy cobweb to a dense mat of mycelium. The white colour can turn pink or even red with age.
Epidemiology: High relative humidity and temperature encourage the disease. Spread is mainly by conidia. The pathogen is a soil inhabiting fungus and is normally introduced into the crop by soil contamination, spores, mycelium on crop debris or by farm workers Spores can easily spread by air movement, workers hands, tools and clothing and by water splash. A high RH and temperature range of 19-22°C results in maximum yield loss.
Control
● Regular cleaning, removal of cut mushroom stems and young half dead mushrooms after each break. Controlling temperature and humidity helps in controlling the disease.
● Annual disinfection of houses and surrounding areas with 2% bordeaux mixture or with 5% formalin solution or fumigation with 2.0-2.5 L formalin and 0.5-1.0 kg chlorinated lime/100 m3 is helpful in controlling disease. Immediate spray after casing with benomyl @ 0.1% also controls the disease. Single application of prochloraz manganese complex (sporgon) at 1.5 g a.i./m2 of bed 9 days after casing gives satisfactory control of the diseases.
iv. Green mould (Trichoderma viride, T.hamatum, T.harzianum, T.koningii, Penicillium cyclopium, Aspergillus spp.).

Fig. 13. Green mould
Different species of Trichoderma have been reported to be associated with green mould symptoms in compost, on casing soil, in the spawn bottles and on grains after spawning. A dense, pure white growth of mycelium may appear on casing surface or in compost, which resembles the mushroom mycelium. Later on mycelial mat turns to green colour (Fig. 13) because of heavy sporulation of causal agent, which is a characteristic symptom of the disease. Thereafter, the mould creeps to surface of casing layer and infects the new parts and developing newly borne primordia. Mushrooms developing in or near this mycelium are brown, may crack and distort, and the stipe peels in a way similar to mushrooms attacked by Verticillium fungicola causing dry bubble disease. Some species induce brownish lesions / spots on caps which may cover the entire cap surface under congenial conditions.
Epidemiology: Green mould generally appears in compost rich in carbohydrates and deficient in nitrogen. If during phase II the compost is trampled too hard in the beds, or the filling weight is too high, this can make the peak heating/ pasteurization difficult. This is certainly the case with compost, which has a short texture and which might also have too high moisture content, resulting in improper pasteurization and conditioning of compost. Frequent use of formalin also tends to promote the development of green moulds. Different sources of primary inoculum of Trichoderma spp. could be dust particles, contaminated clothings, animal vectors especially the mite, Pygmephorus mesembrinae and sciarid flies, air-borne infection, infected spawn, surface spawning, contamination 166 Mushrooms : Cultivation, Marketing and Consumption of compost by handling, machinery and equipments at the mushroom farm. High relative humidity accompanied by a low pH in the casing soil also promotes the development of
Trichoderma spp.Control
● Very good hygiene
● Proper pasteurization and conditioning of compost
● Sterilizing the supplements before use and mixing them thoroughly preferably
after spawning
● Using the correct concentration of formalin (maximum 2%)
● Weekly sprays of mancozeb (0.2%) or bavistin (0.1%) or treatment with zineb
dust gives effective control of the disease
2. Oyster mushroom (Pleurotus spp.)
a. Competitor moulds/weed moulds
Different fungi occurring in the substrate and competing with mushroom mycelium for space and nutrition are: Arthrobotrys sp., Aspergillus niger, A. flavus, A. fumigatus, Alternaria alternata, Cephalosporium aspermum, C. acremonium, Chaetomium globosum, Cladosporium cladosporoides, Coprinus retirugis, C. sterguilinus, Coprinus spp., Cochliobolus specifer, Drechslera bicolor, Fusarium moniliforme, Momniella echinata, Mucor sp., Penicillium sp., Rhizopus oryzae, R. stolonifer, Stachybotrys chartarum, Stilbum nanum, Stysanus medius, Sclerotium rolfsii, Sordaria fimicola, Oedocephalum globerulosum, O.lineatum, Trichoderma viride, Trichothecium roseum Trichurus terrophilus and Phialospora sp. Loss in yield in different Pleurotus spp. by these competitor moulds has been reported upto 70%. In addition to these moulds being competitive, some produce metabolites, which directly inhibit the growth of mushroom mycelium. Most of the competitor moulds have been reported to be completely inhibited under in vitro and/or in vivo conditions by benomyl (50 ppm), carbendazim + blitox (100 ppm each) and Thiram (100 ppm). If proper pasteurization/ sterilization procedures are adopted and bags are incubated at right temperature, recommended bag size are adopted, fresh substrate with right pH after treatment is used and proper hygiene is maintained during spawning and cropping, the mould incidence can be minimized. Many of these appear either by negligence of above-mentioned attributes or if we keep the crop for prolonged period and expose the crop to too high temperature.
b. Fungal diseases
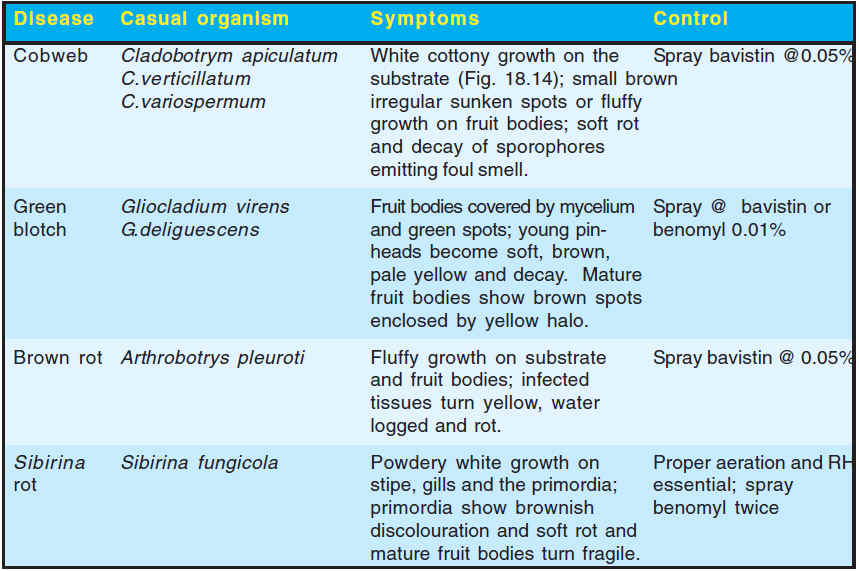
There are four fungal diseases reported on oyster mushroom from India. Their causal agents, symptoms and control measures are presented in Table 2.
Table 2. Fungal diseases of oyster mushrooms in India
3. Paddy straw mushrooms (Volvariella spp.)
Paddy straw mushroom is infected by a number of destructive diseases/competitor moulds like Mycogone perniciosa, Scopulariopsis fimicola and Verticillium spp. in other countries. In India, large number of competitor moulds and few diseases has been reported on this mushroom. Chaetomium spp., Alternaria sp., Coprinus spp. and Sordaria sp. are the most commonly observed contaminants. A ‘button-rot’ disease caused by Sclerotium Cobweb Cladobotrym apiculatum White cottony growth on the Spray bavistin @0.05% C.verticillatum substrate (Fig. 14); small brown C.variospermum irregular sunken spots or fluffy growth on fruit bodies; soft rot and decay of sporophores emitting foul smell. Green Gliocladium virens Fruit bodies covered by mycelium Spray @ bavistin or blotch G.deliguescens and green spots; young pin- benomyl 0.01% heads become soft, brown, pale yellow and decay. Mature fruit bodies show brown spots enclosed by yellow halo. Brown rot Arthrobotrys pleuroti Fluffy growth on substrate Spray bavistin @ 0.05% and fruit bodies; infected tissues turn yellow, water logged and rot. Sibirina Sibirina fungicola Powdery white growth on Proper aeration and RH rot stipe, gills and the primordia; essential; spray primordia show brownish benomyl twice discolouration and soft rot and mature fruit bodies turn fragile.

Fig. 14. Cobweb of oyster mushroom
sp. and bacterial ‘button-rot’ have also been recorded. Combination of insecticide, fungicide and antibiotic (Malathion 0.025% + Dithane Z-78 or benomyl 0.025% + tetracycline 0.025%) are recommended for the management of pests and diseases. Several other competitor moulds namely, Coprinus aratus, C. cinereus, C. lacopus, Psathyrella sp., Penicillium spp., Aspergillus spp., Rhizopus sp., R. nigricans and Sclerotium spp. have been reported from the substrate. Partial sterilization of the straw and sprays on the beds with captan and zineb (0.2%) has been recommended for reducing the damage. Rhizoctoria solani has been recorded on the substrate, which reduces the sporophore formation and causes malformation of fruiting primordia. Considering the rate of growth of paddy straw mushroom and short cropping cycle, disease normally do not reach unmanageable proposition.
4. Other mushrooms
Sporadic attempts have been made to cultivate few other mushrooms like giant mushroom (Stropharia rugoso-annulata), black ear mushroom (Auricularia polytricha), shiitake (Lentinula edodes) and white milky mushroom (Calocybe indica) in different parts of the country and the competitor moulds/diseases recorded on them are briefly mentioned below:
Mycogone rosea was observed parasitizing S. rugoso-annulata under natural conditions. The main symptoms are white cottony growth on gills, light brown spots on stipe and deformity of the sporophores. Cladobotryum verticillatum has been reported on Auricularia polytricha producing white fluffy growth on substrate and fruit bodies resulting in 9-96% yield loss. Carbendazim (0.05%) spray has been reported to be effective for controlling the disease. Trichoderma viride, Trichoderma sp., Aspergillus spp. and Fusarium sp. are commonly recorded as competitors during the cultivation of winter ear mushroom. During the cultivation of C.indica, several competitor moulds namely, Aspergillus niger, A. flavus, A. fumigatus, Rhizopus stolonifer, Mucor sp., S. rolfsii, T. viride, T. hamatum, Fusarium spp. and Coprinus spp. have been isolated from the substrate.
B. Bacterial Diseases
Bacterial diseases have been reported to infect A. bisporus, A. bitorquis, Pleurotus sp., Volvariella sp., Lentinula edodes, Flammulina velutipes and Auricularia sp. The bacterial pathogens induce varieties of symptoms like blotch, mummy, pit, drippy gill, soft rot, yellowing, etc. The bacterial blotch of button mushroom is most important and the same is described here:
Bacterial blotch (Pseudomonas tolaasii): Bacterial blotch of white button mushroom is characterized by brown spots or blotches on the caps and in more severe cases, on the stipes. The most characteristic symptom of bacterial blotch is the occurrence of dark brown areas of blotches on the surface of the cap. These may be initially light in colour but may eventually become dark brown. Severely affected mushrooms may be distorted and the caps may split where the blotch symptoms occur. The enlargement of the spots on the cap surface is dependent upon environmental conditions and is favoured by temperature of at least 20oC together with the presence of water film. The bacterial blotch spots are slimy.
Epidemiology: Casing and dust are the primary source of inoculum for the blotch pathogen into a mushroom house. Even after pasteurization the bacterial pathogen is present in most casing materials. Occurrence of the disease is associated with the rise in the bacterial population on the mushroom cap rather than in the casing. Bacteria present on mushroom surface reproduce in moist conditions especially when moisture or free water film persists for more than 3 hours. Once the pathogen has been introduced at the farm, it may survive between crops on the surfaces, in debris, on tools and on various other structures. When the disease is present on the farm, its secondary spread may take place through workers, implements, ingredients, mushroom spores, debris, etc. Sciarids and mites are also important carriers of the pathogen besides water splashes.
Control
● Manipulation of relative humidity, temperature, air velocity and air movement are of great significance in managing the disease. Temperature above 20oC and relative humidity of more than 85 per cent should be avoided. Additional ventilation and air circulation after watering can ensure quick drying of mushrooms. Temperature fluctuations at higher relative humidity leading to water condensation must be avoided.
● Application of bleaching powder @ 0.15% is effective in managing the disease.
C. Viral Diseases
Viruses infect button, oyster, shiitake, paddy straw and winter mushrooms, however; dieback of button mushroom is the most serious disease.
1. Die back
Mycelium does not permeate or hardly permeates the casing layer or disappears after the normal spread. Mushrooms appear only in dense clusters, maturing too early.
Mycelium isolated from diseased fruit bodies shows a slow and degenerated growth as compared with healthy mycelium. The delayed appearance of the pinheads in the first
flush and formation of fruiting primordia below the surface of the casing layer can be an important indication of the disease. These mushrooms appear above the casing soil
with open pilei. Symptoms on sporophores are highly variable and they include slow development, abnormal mushrooms, watery stipes, brown stipes and drum stick type
fruit bodies. Several viruses of different shapes and sizes are associated with die back disease. In India, viruses measuring 29 nm and 35 nm in diameter have been found to
be associated with a virus disease of button mushroom. Transmission of viruses takes place through mycelium and spores.
Management
● At the termination of the crop, cook out of the compost for 12 hours at 700C and to remove the compost quickly
● Spray the wood with 2 % sodium pentachlorophenate and 1.0 % sodium carbonate
● Disinfect doors, little holes in the floor, shutters, racks, floors and walls with formalin (2 %). Also clean the compost yard and surroundings with formaldehyde
● Use of proper filters during phase II operation of the composting
● Immediately after spawning, spray malathion@ 0.05% and cover the compost with paper. Spray 1 % formalin weekly on the newspaper sheets during spawn run
● Quickly remove cuttings/litter and destroy
The entire farm and its surroundings should be maintained very clean. In the working corridor formalin (2%) should be sprayed. Machines, refrigerator and other utilities should
be disinfected with formalin ( 2%) solution.