
| Oyster Mushroom Cultivation |
Technology for hybrid seed production |
Oyster mushroom commonly referred as ‘Dhingri’ in India, is a basidiomycetes and belongs to the genus ‘Pleurotus’. It is lignocellulolytic fungus that grows naturally in the temperate and tropical forests on dead, decaying wood logs, sometimes on drying trunks of deciduous or coniferous trees. It can also grow on decaying organic matter. The fruit bodies of this mushroom are distinctly shell, fan or spatula shaped with different shades of white, cream, grey, yellow, pink or light brown depending upon the species. However, the colour of the sporophores is extremely variable character influenced by the temperature, light intensity and nutrients present in the substrate. The name Pleurotus has its origin from Greek word, ‘Pleuro’ that means formed laterally or lateral position of the stalk or stem.
The oyster mushroom is one of the most suitable fungal organism for producing protein rich food from various agrowastes without composting. This mushroom is cultivated in about 25 countries of far-east Asia, Europe and America. It is the 3rd largest cultivated mushroom in the world. China alone contributes 88% of the total world production. The other major oyster producing countries are South Korea, Japan, Italy, Taiwan, Thailand and Philippines. At present India produces annually 10,000 tons of this mushroom. It is popularly grown in the states of Orissa, Karnataka, Maharashtra, Andhra Pradesh, Madhya Pradesh, West Bengal and in the North-Eastern States of Meghalaya, Tripura Manipur, Mizoram and Assam.
A. Advantages of Growing Oyster MushroomPleurotus mushroom can degrade and grow on any kind of agricultural or forest wastes, which contain lignin, cellulose and hemicellulose.
2. Choice of speciesAmong all the cultivated mushrooms Pleurotus has maximum number of commercially cultivated species suitable for round the year cultivation. Moreover, variation in shape, colour, texture, and aroma are also available as per consumer’s choice.
3. Simple Cultivation TechnologyPleurotus mycelium can grow on fresh or fermented straw and it does not require composted substrate for growth. Substrate preparation for oyster mushroom is very simple. Further this mushroom does not require controlled environmental conditions like A. bisporus as most of the species have very wide temperature, relatively humidity and CO2 tolerance.
4. Longer shelf lifeUnlike white button mushroom, the oyster mushroom fruit bodies can be easily dried and stored. Dried oyster mushrooms can be instantly used after soaking in hot water for 5 to 10 minutes or it can be used in powdered form for several preparations. Fresh mushrooms have a shelf life of 24-48 h even at room temperature.
5. Highest productivityThe productivity of oyster mushroom per unit time is very high as compared to all other cultivated mushrooms. One can harvest minimum of about 500 to 700 kg of fresh oyster mushroom from one ton of dry wheat or paddy straw in 45-60 days, while with the same quantity of straw only about 400-500 kg of white button mushrooms are obtained in 80-100 days (including period needed for compost preparation). Yield of this mushroom can further be increased by supplementing the substrate with suitable nitrogen source viz., soybean and cottonseed meal or by introducing high yielding cultures/strains.
The present day cultivation technology of oyster mushroom is a result of various successive steps evolved throughout the world during 20th century. A very primitive form of growing Pleurotus spp. was adopted by Lumberman in Europe during 19th century that involved collection of wood logs and stumps showing fructification in nature and keeping them in cool and moist places. First successful experimental cultivation of Pleurotus ostreatus was achieved in Germany by Falck in 1917. In India cultivation of P. flabellatus on paddy straw was reported by Bano & Srivastava in 1962 at CFTRI, Mysore. Kaul and Janardhanan (1970) cultivated a white form of P. ostreatus on dried Euphorbia royleana (Thor) stems. Jandaik and Kapoor in 1974 could grow P. sajor-caju on various substrates including wheat and banana pseudostems.
B. The Biology of Oyster MushroomVisually the basidiocarps or fruit bodies of an oyster mushroom have three distinct parts - a fleshy shell or spatula shaped cap (pileus), a short or long lateral or central stalk called stipe and long ridges and furrows underneath the pileus, called gills or lamellae. The gills stretch from the edge of the cap down to the stalk and bear the spores. If a fruit body is kept on a paper directly (gills facing the paper) a dirty white or lilac deposition of powdery spores can be seen. The spore print colour may be whitish, pinkish, lilac or grey. The spores are hyaline, smooth and cylindrical. The spores are heterothallic and germinate very easily on any kind of mycological media and within 48-96 h whitish thread like colonies could be seen. The mycelium of most Pleurotus spp. is pure white in colour. P. cystidiosus and P. columbinus forms coremia like stalked structures (asexual spores). Basidiospores on germination forms primary mycelium. Fusion between two compatible primary mycelia develops into secondary mycelium, which is having clamp connections and is fertile. Primary mycelium is clampless and non-fertile.
C. Varieties of Oyster MushroomAll the varieties or species of oyster mushroom are edible except P. olearius and P. nidiformis, which are reported to be poisonous. There are 38 species of the genus recorded throughout the world (Singer). In recent years 25 species are commercially cultivated in different parts of the world, which are as follows: P. ostreatus, P. flabellatus, P. florida, P. sajor-caju, P. sapidus, P. cystidiosus, P. eryngii,P. fossulatus, P. opuntiae, P. cornucopiae, P. yuccae, P. platypus, P. djamor, P. tuber-regium, P. australis, P. purpureo-olivaceus, P. populinus, P. levis, P. columbinus, P. membranaceus etc. (Fig. 1).
 |
Fig. 1. Different types of cultivated Pleurotus spp.
The procedure for oyster mushroom cultivation can be divided into following four steps (Fig. 2).
1. Preparation or procurement of spawn
2. Substrate preparation
3. Spawning of substrate and
4. Crop management
The spawn preparation technique for oyster mushroom is similar to white button mushroom (A.bisporus). One should have a pure culture of Pleurotus spp. for inoculation on sterilized wheat grain. It takes 10-15 days for mycelial growth on grains. It has been reported that Jowar and Bajra grains are superior over wheat grains. The mycelium of oyster mushroom grows very fast on wheat grains and 25-30 days old spawn starts forming fruitbodies in the bottle itself. It is, therefore, suggested that the schedule for spawn preparation or spawn procurement should be planned accordingly. Sometimes the mushroom farmers are using active mycelium growing in bags for spawning fresh new oyster mushroom bags. This method can be used on a small scale. There are always chances of spread of contamination through infested straw by active mycelium spawning method so it is not advisable on large scale commercial cultivation.
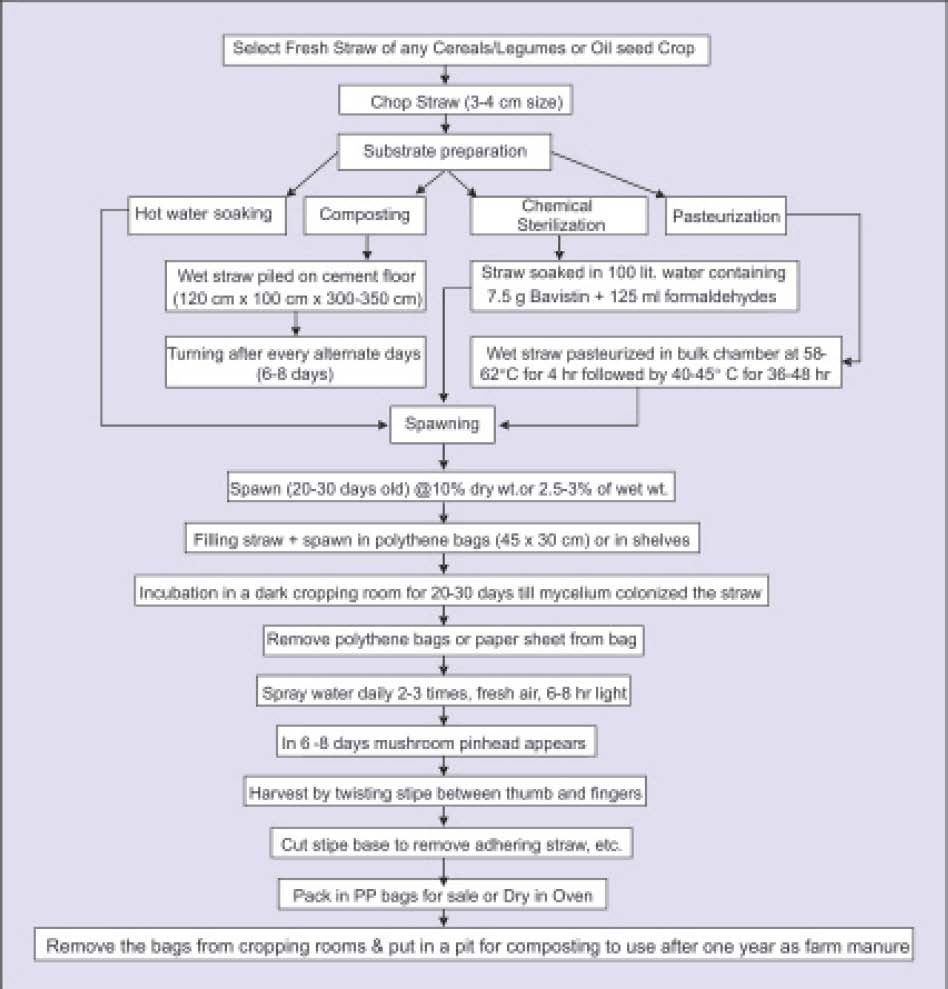 |
Fig. 2. Different steps for oyster mushroom cultivation
2. Substrate preparationA large number of agricultural, forest and agro-industrial by-products are useful for growing oyster mushroom. These by-products or wastes are rich in cellulose, lignin and hemicellulose. However, yield of oyster mushroom largely depends upon the nutrition and nature of the substrate. The substrate should be fresh, dry, free from mould infestation and properly stored. The substrates exposed to rain and harvested immature with green chlorophyll patches inhibit the growth of Pleurotus mycelium due to the presence of competitor moulds. Oyster mushroom can utilize a number of agro-wastes including straws of wheat, paddy and ragi, stalks and leaves of maize, jowar, bajra and cotton, sugarcane bagasse, jute and cotton waste, dehulled corncobs, pea nut shells, dried grasses, sunflower stalks, used tea leaf waste, discarded waste paper and synthetic compost of button mushroom. It can also be cultivated using industrial wastes like papermill sludges, coffee byproducts, tobacco waste, apple pomace, etc. The cellulose and lignin contents are important components of any substrate as far as yield is concerned. Cellulose rich substrates like cotton waste give better yields. Cellulose helps in more enzyme production, which is correlated with higher yield.
b. Methods of substrate preparationThe mycelium of Pleurotus is saprophytic in nature and it does not require selective substrate for its growth. The mycelial growth can take place on a simple water treated straw but there are number of other cellulolytic moulds already present on straw, which compete with Pleurotus mycelium during spawn run and also secrete toxic metabolites hampering its growth. There are various methods to kill undesirable microorganisms present in the straw to favour the growth of Pleurotus mycelium. The popular methods of substrate preparation are as follows.
i. Steam pasteurizationIn this method prewetted straw is packed in wooden trays or boxes and then kept in a pasteurization room at 58-620C for four hours. Temperature of the pasteurization room is manipulated with the help of steam through a boiler. Substrate after cooling at room temperature is seeded with spawn. The entire process takes around 3-5 days. This method is adopted on a commercial scale by Zadrazil and Schneidereit in Germany. There are various minor variations of this method adopted in Europe.
ii. Hot water treatmentThe substrate (wheat staw) after chopping (5-10 cm) is soaked in hot water (65 to 70°C) for one hour or 60 to 120 minutes at 80°C or in case of paddy straw at 85°C for 30- 45 minutes. After draining excess water and cooling, spawn is added. Hot water treatment makes the hard substrate like maize cobs, stems, etc. soft so that the growth of mycelial takes place easily. This method is not suitable for large-scale commercial cultivation.
iii. Chemical sterilization techniqueVarious species of Trichoderma, Gliocladium, Penicillium, Aspergillus and Doratomycs spp. are the common competitor fungi on the straw during oyster mushroom cultivation. If present on the straw during spawn run, they do not allow the growth of mushroom mycelium resulting in yield loss or complete crop failure. When wheat straw or paddy straw is treated by steeping in a chemical solution of carbendazim 50% WP (37.5 ppm) and formaldehyde (500 ppm) for a period of 16-18 h, most of the competitor moulds are either killed or their growth is suppressed for 25-40 days after spawning. The technique, which was standardized at DMR, Solan by Vijay and Sohi in 1987, is as follows: Ninty litres of water is taken in a rust proof drum (preferably of galvanized sheet) or G.I. tub of 200 litres capacity. Ten to twelve kg of wheat straw is slowly steeped in water. In another plastic bucket, Bavistin 7.5 g and 125 ml formaldehyde (37-40%) is dissolved and slowly poured on the already soaked wheat straw. Straw is pressed and covered with a polythene sheet. After 15 to 18 h the straw is taken out and excess water drained. One can use a larger container or cemented tank of 1000-2000 liters for soaking more straw. The chemicals to be added can be calculated as above. The chemical containing water can be reused once again for pasteurization of the straw.
iv. Sterile techniqueIt is also known as Till method. The chopped substrate after soaking in cold water is put in heat resistant polypropylene bags and sterilized in an autoclave at 20 p.s.i. for 1-2 hours followed by spawning under aseptic conditions. This method is more suitable for research work rather than on large-scale commercial production.
v. Fermentation or compostingThis method is a modification of composting technique used for white button mushroom. It is most suitable for hard substrates like cotton stalks, maize stalks, leguminous stubbles, etc. Both aerobic and anaerobic fermentation of the substrate is suitable for Pleurotus cultivation. Composting should be done on a covered area or shed. Chop the substrate into 5-6 cm long pieces. Add ammonium sulphate or urea (0.5-1%) and lime (1%) on dry weight basis of the ingredients. Horse manure or chicken manure (10% dry weight basis) can also be used instead of nitrogenous fertilizers. Addition of lime improves the physical structure of the compost. After mixing all the ingredients sprinkle water till it is completely wet. Prepare a triangular heap of 75-90 cm but not more than 1 meter height. After 2 days of fermentation, turning of pile is done adding 1% superphosphate and 0.5% lime. The compost will be ready after 2 days of this turning. It can be spawned as such or used after pasteurization. The pasteurization of substrate can be done using the tunnels used for button mushroom compost preparation. The straw, after initial fermentation for 4 to 6 days, is filled in tunnel upto 2-3 ft. height and pasteurized at 58-600C for 4 h and conditioned at 40-450C for 30-36 h.
The nitrogen content in most of the substrates ranges between 0.5 to 0.8% and hence addition of organic nitrogen in the straw helps in getting higher yields. Some of the common supplements are wheat bran, rice bran, cottonseed meal, soybean cake, etc. Wheat bran and rice bran should be used @ 10% while cottonseed meal, soybean cake and groundnut cake should be tried @ 3-6% on dry weight basis of the substrate. The supplements should be treated with 25 ppm carbendazim (250 mg in 10 lit. water) for 14- 16 h. Supplements are thoroughly mixed with straw before spawning. Addition of supplements increases substrate temperature by 2-3°C or even more and hence supplementation during summer season is not advisable. However, during winter months though increased temperature is observed, which helps in quick spawn run. Excess nitrogen can attract mould infestation, which should be taken care of.
Freshly prepared (20-30 days old) grain spawn is best for spawning. The spawning should be done in a pre-fumigated room (48 h with 2% formaldehyde). The spawn should be mixed @ 2 to 3% of the wet wt. of the substrate. One bottle spawn of 300 g is sufficient for 10-12 kg of wet substrate or 2.8 to 3 kg of dry substrate. Spawn can be mixed thoroughly or mixed in layers. Spawned substrate is filled in polythene bags (60 x 45 cm) of 125-150 gauze thickness. Ten to 15 small holes (0.5-1.0 cm dia) should be made on all sides especially in the bottom for leaching of excess water (Fig. 3). Perforated bags give higher and early crop (4-6 days) than non-perforated bags because of accumulation of high CO2, which inhibits fruiting. One can also use empty fruit packing cartons or wooden boxes for filling substrate. Polythene sheets of 200-300 gauze thickness are spread in rectangular wooden or metal box, spawned substrate is filled and the polythene sheet is folded from all the four sides to make a compact rectangular box. It is tightly pressed and tied with a nylon rope. The block is incubated as such and after mycelium growth polythene sheet is removed.
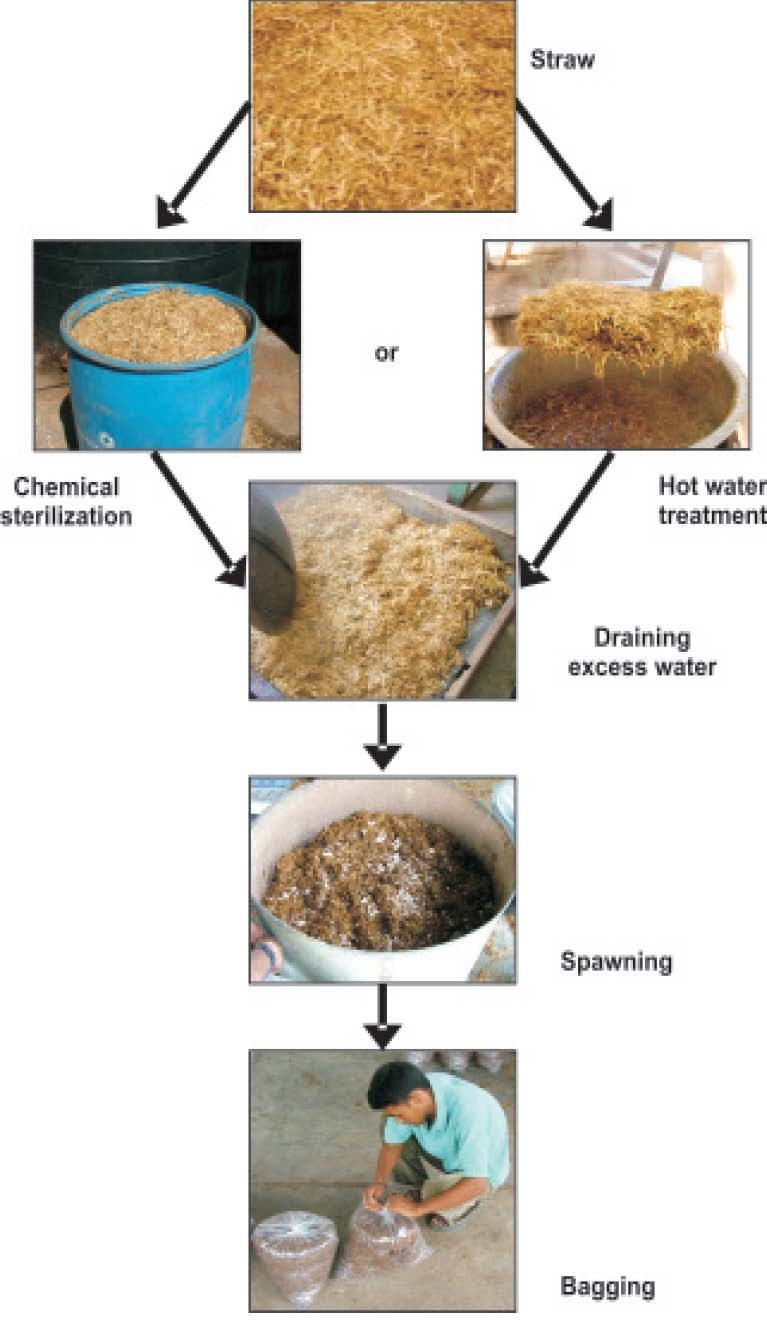 |
Fig. 3. Pictorial flow chart of oyster mushroom cultivation
The spawned bags or blocks are kept in incubation room for mycelial growth.
a. IncubationSpawned bags can be kept on a raised platform or shelves or can be hanged in cropping room for mycelial colonization of the substrate. Although mycelium can grow between 10-30°C but the optimum temperature lies between 22-26°C. Higher temperature (more than 30°C) in the cropping room will inhibit the growth and kill the mycelium. Daily maximum and minimum temperature of cropping rooms and beds should be recorded. The bed temperature is generally 2-4°C higher than the room temperature. Mycelium can tolerate very high CO2 concentration of 15-20%. During mycelial growth the bags are not to be opened and no ventilation is needed. Moreover, there is no need for any high relative humidity, so no water should be sprayed.
b. Fruit body inductionOnce the mycelium has fully colonized the substrate, it forms a thick mycelial mat and is ready for fruiting. Contaminated bags with mould infestation should be discarded while bags with patchy mycelial growth may be left for few more days to complete the spwan run. In no case bags should be opened before 16-18 days except in case of P. membranaceus and P. djamor var. roseus, which forms fruit bodies within 10 days even in closed bags from small holes. Casing is not required in oyster mushroom cultivation. All the bundles, cubes or blocks are arranged on iron/wooden platforms or shelves with a minimum distance of 15-20 cm between each bag in the tier. They can also be hanged. The cultural conditions required for fruiting are as follows :
i. TemperatureMycelial growth of all the Pleurotus spp. can take place between 20-30°C. However, for fruiting different species have different temperature requirement. Depending upon the temperature requirement of a species, they can be categorized into two groupswinter or low temperature requiring species (10-20°C) and summer or moderate temperature requiring species (16-30°C). Summer varieties can fructify at low temperature but the winter varieties will not fruitfy at higher temperatures. They need a low temperature shock for inducing fruit body formation. Commercial varieties, which can be cultivated during summer are P. flabellatus, P. sapidus, P. citrinopileatus and P. sajor-caju. Low temperature requiring species are P. ostreatus, P. florida, P. eryngii, P. fossulatus and P. cornucopiae. We have isolated a wild species of P. cornucopiae, which is suitable for growing between 15-25°C. The growing temperature not only affects the yield but also the quality of produce. The pileus or cap colour of P. florida is light brown when cultivated at low temperature (10-15°C) but changes to white pale to yellowish at 20-25°C. Similarly fruit body colour of P. sajor-caju when cultivated at 15-19°C is white to dull white with high dry matter content while at 25-30°C, it is whitish brown to dark brown with less dry matter.
ii. Relative humidityAll the Pleurotus species require high relative humidity (75-85%) during fruiting. To maintain relative humidity, water spraying is to be done in the cropping rooms. During hot and dry weather conditions daily 2-3 spray are recommended while in hot and humid conditions (monsoon) one light spray will be sufficient. The requirement of water spraycan be judged by touching the surface of the substrate. Spraying should be done with a fine nozzle to create a mist or fog in the cropping room. It is desirable that mushrooms are harvested before water spray. Ventilators and exhaust fans should be operated for air circulation so that the excess moisture from the pileus surface evaporates. Sometimes fruit bodies gives offensive smell due to the growth of saprophytic bacteria on the wet pileus surface, under such conditions 0.05% bleaching powder spray at weekly interval is recommended.
 |
Fig. 4. Round the year cultivation of oyster mushrooms species in North West Himalayas
iii. Oxygen and carbon dioxide requirementsThe oyster mushroom can tolerate high carbon dioxide concentration during spawn run (upto 20,000 ppm or 2%) while it should be less than 600 ppm or 0.06% during cropping. Therefore sufficient ventilation should be provided during fructification. If the CO2 concentration is high, the mushrooms will have long stipe and small pileus. Mushrooms will appear like a mouth of trumpet.
iv. LightUnlike green plants mushrooms do not require light for the synthesis of food. They grow on dead organic plant material. Light is required to initiate fruit body initiation. For primordia formation, light requirement is 200 lux intensity for 8-12 h. Inadequate light conditions can be judged by long stalk (stipe), small cap and poor yield. The colour of the pileus is also influenced by the light intensity and its duration. Fruit bodies raised in bright light are dark brown, grey or blackish coloured. If the light intensity is less than 100 lux the mushrooms will be pale yellowish.
v. Hydrogen ion concentration (pH)The optimum pH during mycelial colonization should be between 6.0 to 7.0 while the pH of the water for spraying should be neither too acidic nor alkaline. Water should not contain harmful salts. Rusted iron drums and tubs used for substrate treatment or storing water for spraying delay fructification due to presence of excess iron in the water.
F. Post Harvest PracticesMushrooms should always be harvested before water spray. The right stage for picking can be judged by the shape and size of fruit body. In young mushrooms, the edge of the cap is thick and cap margin is enrolled while the cap of mature mushroom is flat and inward curling starts. It is advisable to harvest all the mushrooms at one time from a bag so that the next crop of mushrooms starts early. After harvesting lower portion of the stalk with adhering debris should be cut using a knife. Stipe is kept short or almost nonexistent, as it is hard and not liked by many consumers. Fresh mushrooms should be packed in perforated polythene bags for marketing. They can also be sun dried by spreading on a cotton cloth in bright sunlight or diffused light. The dried produce with 2-4% moisture can be stored for 3 to 4 months after proper sealing.
G. Medicinal and Nutritional Value of Oyster MushroomOyster mushrooms are 100% vegetarian and the nutritive value of oyster mushroom is as good as other edible mushrooms like white button mushroom (A. bisporus), shiitake (Lentinula edodes) or paddy straw mushroom (Volvariella spp.). They are rich in vitamin C and B complex. Protein content varies between 1.6 to 2.5% on fresh weight basis. It has most of the mineral salts required by the human body such as potassium, sodium, phosphorus, iron and calcium. The niacin content is about ten times higher than any other vegetables. A polycyclic aromatic compound pleurotin has been isolated from P. griseus, which possess antibiotic properties.